The molar mass of NaOH is 40.00 g/mol, a fundamental concept in chemistry that holds significant importance in various chemical calculations. This guide delves into the definition, derivation, and applications of the molar mass of NaOH, providing a comprehensive understanding of this essential property.
Composed of sodium, oxygen, and hydrogen, the molar mass of NaOH is directly related to the atomic masses of these elements. Its value, 40.00 g/mol, plays a crucial role in determining the mass-to-mole ratios in chemical reactions and serves as a basis for calculating concentrations and dilutions for safe handling.
The Molar Mass of NaOH: The Molar Mass Of Naoh Is 40.00 G/mol

In chemistry, the molar mass of a substance refers to the mass of one mole of that substance. The molar mass is a fundamental property used in various chemical calculations, providing insights into the composition and behavior of substances.
Molar Mass of NaOH
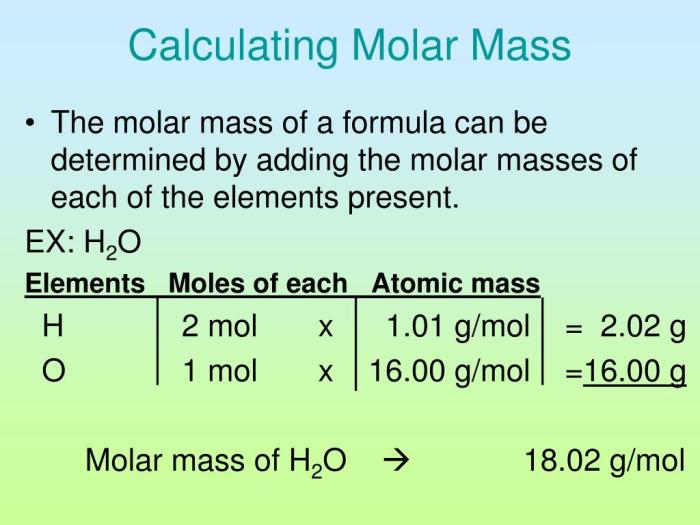
The given value for the molar mass of NaOH is 40.00 g/mol. This value is experimentally determined by measuring the mass of a known number of moles of NaOH and calculating the ratio between the mass and the number of moles.
Composition of NaOH, The molar mass of naoh is 40.00 g/mol
NaOH is composed of three elements: sodium (Na), oxygen (O), and hydrogen (H). The molar mass of NaOH is the sum of the atomic masses of these elements, which are 22.99 g/mol for Na, 16.00 g/mol for O, and 1.01 g/mol for H.
Applications of NaOH
- Soap and detergent production:NaOH is used as a base in the saponification process, where fats and oils are converted into soap.
- Paper manufacturing:NaOH is used to dissolve lignin in the wood pulping process, making paper stronger and whiter.
- Textile industry:NaOH is used in the mercerization process to enhance the strength and luster of cotton fibers.
- Food industry:NaOH is used as a food additive to control acidity and pH levels in various food products.
Calculations Involving NaOH Molar Mass
The molar mass of NaOH can be used to convert between mass and moles in chemical reactions. For example, to calculate the number of moles of NaOH in 10.00 g of NaOH, we can use the following formula:
| Mass (g) | Molar Mass (g/mol) | Moles (mol) |
|---|---|---|
| 10.00 | 40.00 | 0.250 |
Safety Considerations
NaOH is a corrosive substance that can cause severe burns. When handling NaOH, it is essential to wear appropriate protective gear, including gloves, goggles, and a lab coat. The molar mass of NaOH can be used to calculate appropriate dilutions or concentrations for safe use, ensuring that the substance is handled responsibly.
Clarifying Questions
What is the molar mass of NaOH?
The molar mass of NaOH is 40.00 g/mol.
How is the molar mass of NaOH determined?
The molar mass of NaOH is determined by adding the atomic masses of sodium, oxygen, and hydrogen atoms present in its formula.
What are the applications of NaOH?
NaOH is widely used in various industries, including soap and detergent manufacturing, paper production, and water treatment.