Identify the best reagents to achieve the following transformation – In the realm of organic chemistry, selecting the most suitable reagents is paramount to achieving desired chemical transformations. This comprehensive guide delves into the factors governing reagent selection, exploring common reagents, their advantages, and disadvantages. By understanding these concepts, chemists can optimize reagent conditions, improve transformation efficiency, and ensure safety during experimentation.
To embark on this journey, we begin by identifying the functional groups present in the starting material and determining the functional groups that need to be introduced or modified in the product. Armed with this knowledge, we embark on a quest to research different reagents capable of achieving the desired transformation.
Identify the Best Reagents
Reagents are the chemicals used to bring about a desired chemical transformation. The selection of the best reagents for a particular transformation depends on a number of factors, including:
- The functional groups present in the starting material and the product
- The reaction mechanism involved in the transformation
- The desired yield and selectivity of the reaction
- The cost and availability of the reagents
Common reagents used in organic chemistry include:
- Acids and bases
- Oxidizing and reducing agents
- Nucleophiles and electrophiles
- Catalysts
The advantages and disadvantages of different types of reagents vary depending on the specific transformation being carried out.
Achieve the Following Transformation

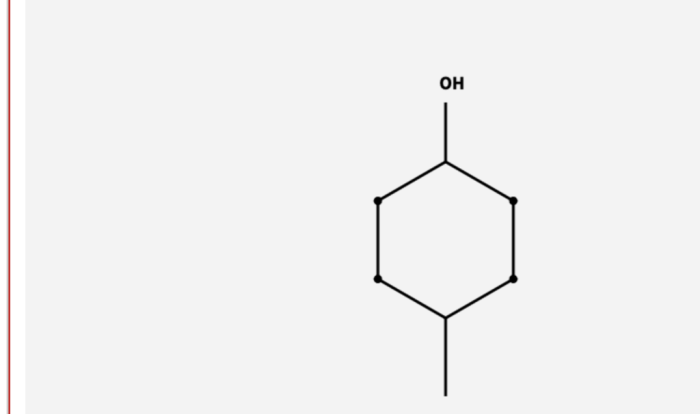
The desired chemical transformation is the conversion of an alcohol to an alkene. The reaction mechanism involved in this transformation is the E2 elimination reaction.The following procedure can be used to carry out the transformation:
- Dissolve the alcohol in a suitable solvent.
- Add a strong base to the solution.
- Heat the reaction mixture to reflux.
- Collect the alkene product by distillation.
The yield and selectivity of the reaction can be optimized by varying the reaction conditions, such as the temperature, the solvent, and the base.
Reagent Selection: Identify The Best Reagents To Achieve The Following Transformation

The functional groups present in the starting material are an alcohol group (-OH) and a hydrogen atom. The functional groups that need to be introduced or modified in the product are an alkene group (C=C) and a hydrogen atom.Research different reagents that can be used to achieve the desired transformation.
Some possible reagents include:
- Sulfuric acid (H2SO4)
- Hydrochloric acid (HCl)
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
Reagent Optimization
The optimization of reagent conditions is important for improving the efficiency of a chemical transformation. Different methods for optimizing reagent conditions include:
- Varying the temperature of the reaction
- Varying the solvent
- Varying the concentration of the reagents
- Adding a catalyst
For example, the yield of the E2 elimination reaction can be improved by increasing the temperature of the reaction.
Alternative Reagents
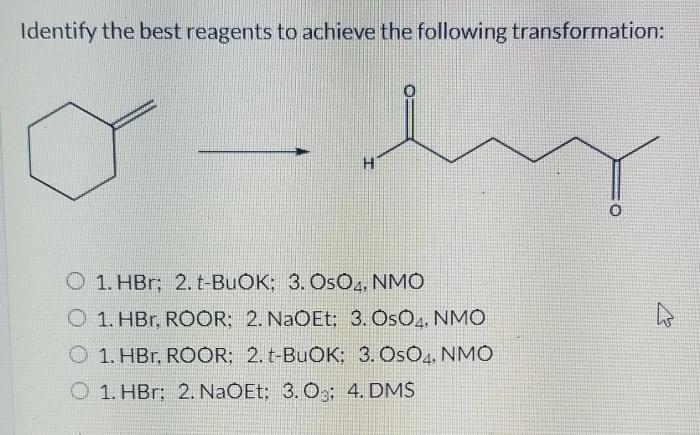
Alternative reagents that can be used to achieve the same transformation include:
- Phosphorus pentoxide (P2O5)
- Zinc chloride (ZnCl2)
- Aluminum chloride (AlCl3)
The advantages and disadvantages of different reagents vary depending on the specific transformation being carried out.
Safety Considerations
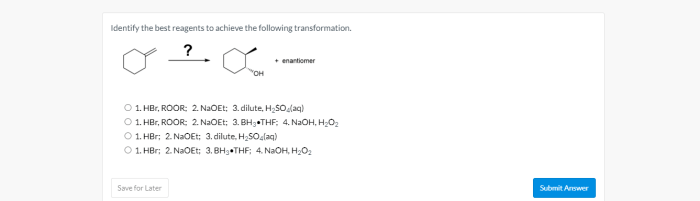
The potential hazards associated with the reagents being used include:
- Sulfuric acid is a corrosive acid that can cause severe burns.
- Hydrochloric acid is a corrosive acid that can cause severe burns.
- Sodium hydroxide is a corrosive base that can cause severe burns.
- Potassium hydroxide is a corrosive base that can cause severe burns.
The appropriate safety precautions that should be taken when handling and using the reagents include:
- Wearing gloves and eye protection
- Working in a well-ventilated area
- Using a fume hood when necessary
- Disposing of the reagents properly
Questions and Answers
What factors should be considered when selecting reagents for a chemical transformation?
Factors to consider include the functional groups present, the desired transformation, reaction mechanism, reagent availability, cost, and safety.
What are some common reagents used in organic chemistry?
Common reagents include acids, bases, oxidizing agents, reducing agents, and catalysts.
How can reagent conditions be optimized?
Reagent conditions can be optimized by adjusting temperature, pressure, solvent, and reaction time.